Draw The Main Lewis Structure Of Nof
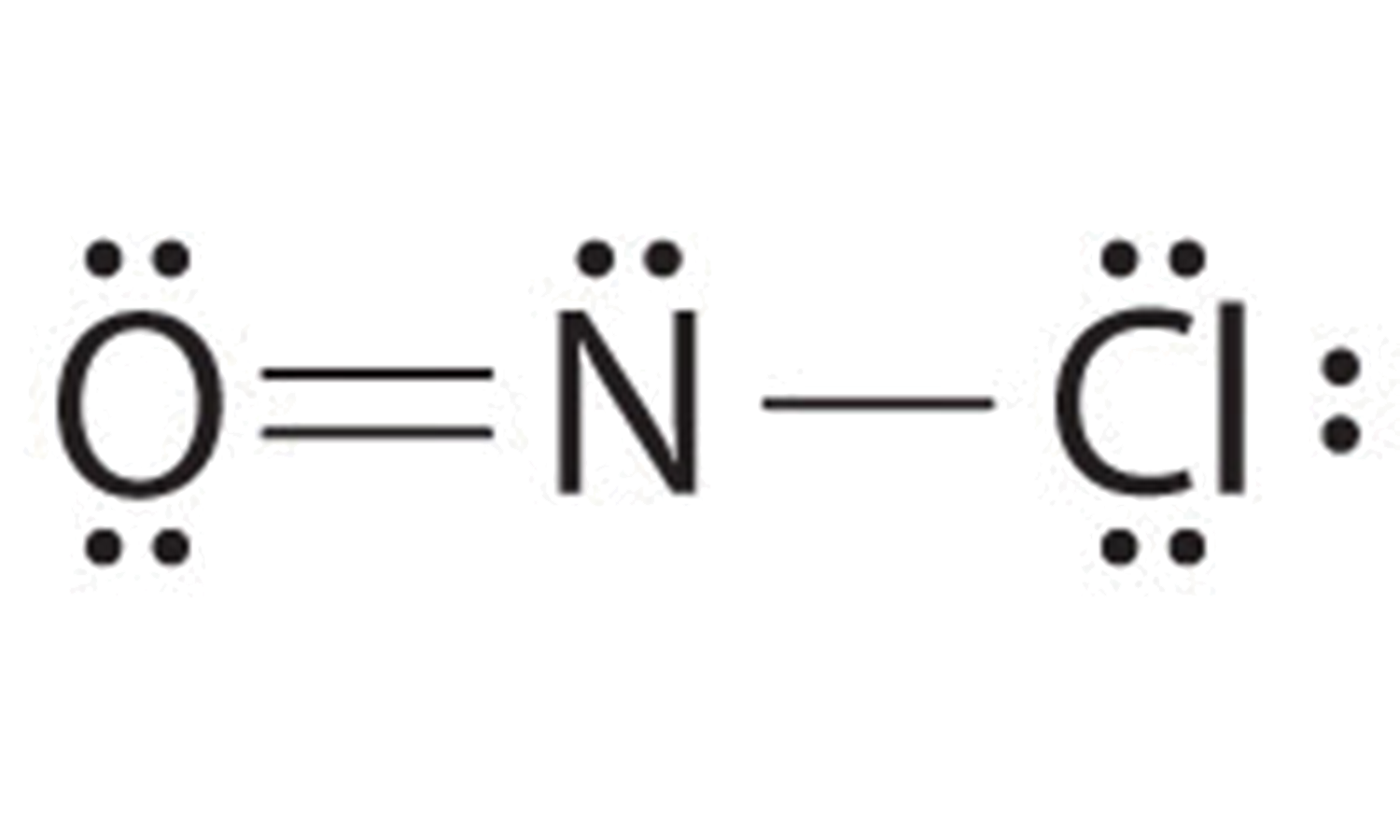
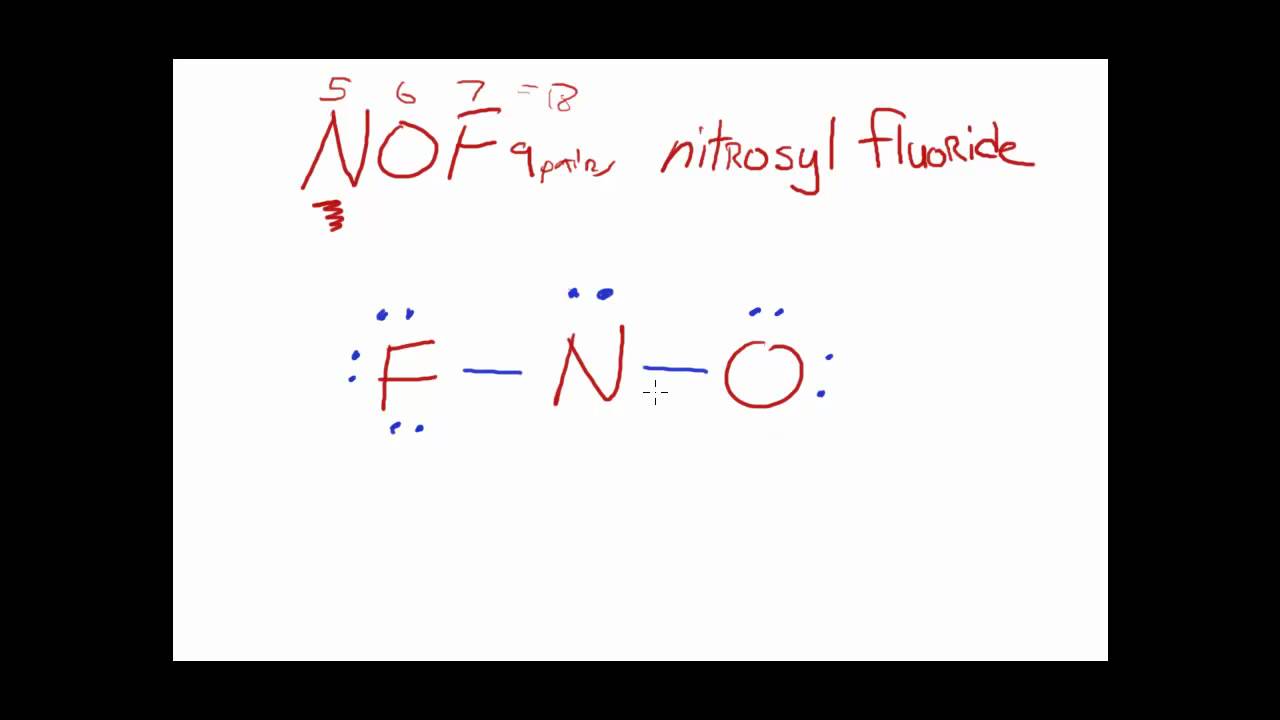
Draw The Main Lewis Structure Of Nof - Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The nof lewis structure is very similar to nocl and nobr. Web nof is a chemical formula for nitrosyl flouride. Web lewis structure of nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Can youu imagine learning how to draw lewis structures in less than 60 seconds? The lewis structure of nof is drawn by determining the total valence electrons, arranging the atoms with the least electronegative in the center, depicting. Web the second structure requires more work. Web steps of drawing nof lewis structure step 1: Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine.the nitrogen is double bonded to the oxygen atom on one sidea. The basic idea is to draw. Use these steps to correctly draw the nof lewis structure: In the nof lewis structure nitrogen (n) is the least. In order to find the total valence electrons in a nof molecule, first of all you. Draw the main lewis structure of nof. In order to draw the lewis. Web a plot of the potential energy of the system as a function of the internuclear distance (figure 5.3.2 ) shows that the system becomes more stable (the energy of the system. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. In your lab notebook, draw a large picture (lewis structure) of all the molecules (such. Can youu imagine learning how to draw lewis structures in less than 60 seconds? Web it is possible to draw a structure with a double bond between a boron atom and a fluorine atom in bf 3, satisfying the octet rule, but experimental evidence indicates the bond. Draw the main lewis structure of nof. Part b draw the main lewis. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The lewis structure of nof is drawn by determining the total valence electrons, arranging the atoms with the least electronegative in the center, depicting. Web 6. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. Draw the main lewis structure of nof. Use these steps to correctly draw the nof lewis structure: Web drawing the lewis structure for nof. Web lewis structure of nof. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. Web the second structure requires more work. The basic idea is to draw. Find the total valence electrons in nof molecule. Web the main purpose of lewis structures is to show the arrangement of valence electrons around atoms in a molecule or compound. Draw the main lewis structure of nof. Understand how atoms bond in nof and their unique. In order to find the total valence electrons in a nof molecule, first of all you. Web drawing the lewis structure for nof. Web nof is a chemical formula for nitrosyl flouride. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web the second structure requires more work. Web nof is a chemical formula for nitrosyl flouride. Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine.the nitrogen is double bonded to the oxygen atom on one sidea. In order to. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Web nof is a chemical formula for nitrosyl flouride. The lewis structure of nof is drawn by determining the total valence electrons,. Draw the main lewis structure of nof. Determine the number of bonding. In order to draw the lewis. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Web drawing the lewis structure for nof. Web the second structure requires more work. In your lab notebook, draw a large picture (lewis structure) of all the molecules (such. In order to find the total valence electrons in a nof molecule, first of all you. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. Can. Therefore, we recommend that when you draw a structure that satisfies the octet rule, you stop there without adding more bonds. Part b draw the main lewis structure of nof. Understand how atoms bond in nof and their unique. In the nof lewis structure nitrogen (n) is the least. Use these steps to correctly draw the nof lewis structure: Understand how atoms bond in nof and their unique. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the. Web draw the main lewis structure of nof. Determine the number of bonding. Web it is possible to draw a structure with a double bond between a boron atom and a fluorine atom in bf 3, satisfying the octet rule, but experimental evidence indicates the bond. Find the total valence electrons in nof molecule. Draw the main lewis structure of nof. The basic idea is to draw. Calculate the total number of valence electrons. In the nof lewis structure nitrogen (n) is the least. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Let’s apply the concepts we have learned. Web a plot of the potential energy of the system as a function of the internuclear distance (figure 5.3.2 ) shows that the system becomes more stable (the energy of the system. Draw the main lewis structure of nof. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. The lewis structure of nof is drawn by determining the total valence electrons, arranging the atoms with the least electronegative in the center, depicting.Main Lewis Structure Of Nof
How To Draw The Main Lewis Structure Of Nof learnpedia.click
NOF Lewis Structure, Geometry, Hybridization, and Polarity
Draw the main lewis structure of nof. draw nonbonding electrons using
Lewis Structure
Structure and Geometry The NOF example YouTube
Nof Lewis Structure And Resonance Structures
Draw The Main Lewis Structure Of Nof.
NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube
NOF Lewis Structure How to Draw the Lewis Structure for NOF (Nitrosyl
Web The Main Purpose Of Lewis Structures Is To Show The Arrangement Of Valence Electrons Around Atoms In A Molecule Or Compound.
In Your Lab Notebook, Draw A Large Picture (Lewis Structure) Of All The Molecules (Such.
Lewis Structure As We Already Know Is The Pictorial Representation Of Electrons Around The Atoms In A Molecule.
Here, The Given Molecule Is Nof.
Related Post: